|
Chapter |
†††††††††††††† Despite the hydrogen atomís overall neutrality, hydrogen does have an internal distribution of electric charge.
As we know that the simplest hydrogen
atom consists of one proton and one electron, the hydrogen atom as total has
zero electric charge. Combine both electron charge distribution and proton
charge distribution together, then we can get the hydrogen atomís electric charge
distribution as follows:
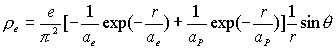 †††††††††††††† †††††††††††††††††††††††††††††††(17.1)
†††††††††††††† †††††††††††††††††††††††††††††††(17.1)
Hydrogen atom
magnetic charge density distribution equation:
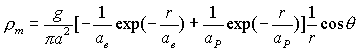 ††††††††††††††††††††††††††††††††††† ††††(17.2)
††††††††††††††††††††††††††††††††††† ††††(17.2)
Hydrogen atom
electric charge distribution equation:
![]() †††††† †††††††††††††††††(17.3)
†††††† †††††††††††††††††(17.3)
Hydrogen atom
magnetic charge distribution equation:
![]() †††††††††††††††††††††††††††††† (17.4)
†††††††††††††††††††††††††††††† (17.4)
Hydrogen atom
electric field equation:
![]() †††††††††††††††††††††††††
††††††(17.5)
†††††††††††††††††††††††††
††††††(17.5)
Hydrogen atom magnetic
field equation:
![]() ††††††††††††††††††††††
†††††††††††(17.6)
††††††††††††††††††††††
†††††††††††(17.6)
Base on these equation,
we can get hydrogen magnetic field energy:
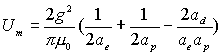 ††††††††††††††††††††††††††††††††† ††††††††††††††††††††††††††††††††††††††††(17.7)
††††††††††††††††††††††††††††††††† ††††††††††††††††††††††††††††††††††††††††(17.7)
In which
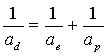 ††††††††††††††††††††††††††††††††††††††††††††††††† †††††††††††††††††††††††††††††††††††††††††† ††††††††††(17.8)
††††††††††††††††††††††††††††††††††††††††††††††††† †††††††††††††††††††††††††††††††††††††††††† ††††††††††(17.8)
Thus:
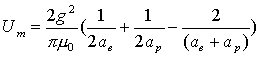 ††††††††††††††††††††††††††††††††† †††††††††††††††††††† ††††††††††(17.9)
††††††††††††††††††††††††††††††††† †††††††††††††††††††† ††††††††††(17.9)
Thus:
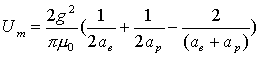 ††††††††††††††††††††††††††
††††††††††††††††††††††††††† ††††††††(17.10)
††††††††††††††††††††††††††
††††††††††††††††††††††††††† ††††††††(17.10)
Thus:
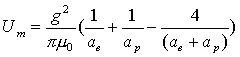 ††††††††††††††††††††††††††††††††† ††††††††††††† ††††††††††††††††††††(17.11)
††††††††††††††††††††††††††††††††† ††††††††††††† ††††††††††††††††††††(17.11)
The hydrogen
electric field energy is:
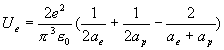 †††††††††††††††††††††††††††††††††††††† †††††††††††† ††††††††††††††††††††(17.12)†††
†††††††††††††††††††††††††††††††††††† †††††††††††††††††††††††
†††††††††††††††††††††††††††††††††††††† †††††††††††† ††††††††††††††††††††(17.12)†††
†††††††††††††††††††††††††††††††††††† †††††††††††††††††††††††
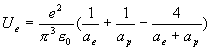 †††††††††††††††††††††††††††††††††††††††††††† ††††††††††††††††††††† ††††††††(17.13)†††††††††††††††††††††††† ††††††††††††††† †††††††††††
†††††††††††††††††††††††††††††††††††††††††††† ††††††††††††††††††††† ††††††††(17.13)†††††††††††††††††††††††† ††††††††††††††† †††††††††††
Thus, the hydrogen
atomís electromagnetic energy is:
†
![]() †††††††††††††††††††††††††††††† ††††††††††††††††††††††††† ††††††††††††††††††††††††††††††††††††††††††††(17.14)
†††††††††††††††††††††††††††††† ††††††††††††††††††††††††† ††††††††††††††††††††††††††††††††††††††††††††(17.14)
Thus:
![]() ††††††††††††††††††††††††††††††††† †††††††††††††††††††††† ††††††(17.15)
††††††††††††††††††††††††††††††††† †††††††††††††††††††††† ††††††(17.15)
As we know
![]() ††††††††††††††††††††††††††††††††† ††††††††††††††††††††††† ††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††(17.16)
††††††††††††††††††††††††††††††††† ††††††††††††††††††††††† ††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††(17.16)
Thus:
![]() ††††††††††††††††††††††††††††††††† ††††††††††††† ††††††††††††††††††††††††††††††††††††††††††(17.17)
††††††††††††††††††††††††††††††††† ††††††††††††† ††††††††††††††††††††††††††††††††††††††††††(17.17)
Thus:
![]() ††††††††††††††††††††††††††††††††† ††††††††††††††††††††††† ††††††††††††††††††††††††††††††††††††††††††(17.18)
††††††††††††††††††††††††††††††††† ††††††††††††††††††††††† ††††††††††††††††††††††††††††††††††††††††††(17.18)
As we know:
![]() †††††††††††††††††††††††††
††††(17.19)
†††††††††††††††††††††††††
††††(17.19)
![]() †††††††††† ††††††††††††††††††(17.20)
†††††††††† ††††††††††††††††††(17.20)
Thus:
![]() ††††††††††††††††††††††††††††††††† †††† ††††††††(17.21)
††††††††††††††††††††††††††††††††† †††† ††††††††(17.21)
Thus, the momentum
density for hydrogen is:
![]() ††††††††††††††††††††††††††††††††† ††† ††††††††††††††††††††(17.22)
††††††††††††††††††††††††††††††††† ††† ††††††††††††††††††††(17.22)
The energy density
for hydrogen is:
![]() † ††††††††††††††††††††††††††††††††††† ††††††††††††††††††††††††††††††††††††††††††††††††††††††††(17.23)
† ††††††††††††††††††††††††††††††††††† ††††††††††††††††††††††††††††††††††††††††††††††††††††††††(17.23)
![]() ††††††††††††††††††††††††††††††††† ††(17.24)
††††††††††††††††††††††††††††††††† ††(17.24)
Thus, the velocity
of a hydrogen atom is:
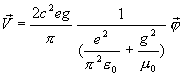 ††††††††††††††††††††††††††††††††† ††† ††††††††††††††††††††††††††††††††††††††††††††(17.25)
††††††††††††††††††††††††††††††††† ††† ††††††††††††††††††††††††††††††††††††††††††††(17.25)
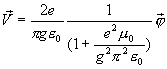 †† †††††††††††††††††††††††††††††††††††††† ††††††††††††††††††††††††††††††††††††††††††††(17.26)
†† †††††††††††††††††††††††††††††††††††††† ††††††††††††††††††††††††††††††††††††††††††††(17.26)
Thus:
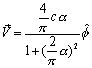 †††††††††††††††††††††††††††††††† ††† ††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††(17.27)
†††††††††††††††††††††††††††††††† ††† ††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††(17.27)
We find out that
inside the hydrogen atom, the velocity is the same as for the electron, which
has a constant speed value.
As we know the field
angular momentum density is:
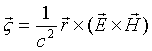 †††††††††††††††††††††††††††††††††† †††††††††††††††††††††† ††††††††††††††††††††††††††††††(17.28)
†††††††††††††††††††††††††††††††††† †††††††††††††††††††††† ††††††††††††††††††††††††††††††(17.28)
Thus, the hydrogen
atom field angular momentum is as follows:
†††††††††††††††††††††††††††††††††††††††††††††††
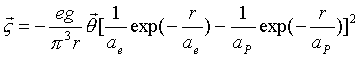 †††††††††††††††† †††††††††††††††††††(17.29)
†††††††††††††††† †††††††††††††††††††(17.29)
Then,
integrating equation (17.28), we can get the field angular momentum:
![]() †††††††††††††††††††††††††††††††† †††††††††††††† ††††††††††††††††††††††††††††††††††††††††††††(17.30)
†††††††††††††††††††††††††††††††† †††††††††††††† ††††††††††††††††††††††††††††††††††††††††††††(17.30)
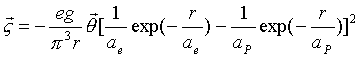 †††††††††††††††† †††††††††††††††††††(17.31)
†††††††††††††††† †††††††††††††††††††(17.31)
For the cylindrical
coordinates (![]() ,
, ![]() , z), which has the following relationship with the spherical
coordinate (r,
, z), which has the following relationship with the spherical
coordinate (r, ![]() ,
, ![]() ):
):
![]() †
†
![]()
We can separate the angular momentum density into
z component and ![]() †component,
†component,
The
z component of angular momentum density is:
![]() ††††††††††††††††††††††††††††††† ††††††††††††††††††† (17.32)
††††††††††††††††††††††††††††††† ††††††††††††††††††† (17.32)
The![]() component of angular momentum density is:
component of angular momentum density is:
![]() ††††††††††††††††††††††††††† ††††††††††††††††††† (17.33)
††††††††††††††††††††††††††† ††††††††††††††††††† (17.33)
As
we know, the volume element is:![]()
For
the ![]() †component of angular momentum
density, because
†component of angular momentum
density, because
![]() ††††††††††††††††††††††††††††††† ††††††††††††† ††††††††††††††††††††††††††††††††††††††††††††††† ††††††(17.34)
††††††††††††††††††††††††††††††† ††††††††††††† ††††††††††††††††††††††††††††††††††††††††††††††† ††††††(17.34)
Thus, we can get
the ![]() †component of angular momentum:
†component of angular momentum:
![]() †††††††††††††††††††††††††††††††††† †††††††††††† ††††††††††††††††††††††††††††††††††††††††††††††††††††††††††† ††††††(17.35)
†††††††††††††††††††††††††††††††††† †††††††††††† ††††††††††††††††††††††††††††††††††††††††††††††††††††††††††† ††††††(17.35)
For
the z component of electron angular momentum, we have:
![]() †††††††††††††††††††††††† ††††††† (17.36)
†††††††††††††††††††††††† ††††††† (17.36)
Thus:
![]() †††††††††††††††††††††††††††††††††††† ††††††† (17.37)
†††††††††††††††††††††††††††††††††††† ††††††† (17.37)
Thus:
![]() †††††††††††††††††††††††††††††††††† ††††††† (17.38)
†††††††††††††††††††††††††††††††††† ††††††† (17.38)
Thus:
![]() ††††††††††††††††††††††††††††††††† ††††(17.39)†††††††††††††††††††††††††††††††††††††††† ††††
††††††††††††††††††††††††††††††††† ††††(17.39)†††††††††††††††††††††††††††††††††††††††† ††††
When ![]() , thus the z component of angular momentum is:
, thus the z component of angular momentum is:
![]() ††††††††††††††††††††††††††† †††††††††††††††††††††† ††††††(17.40)
††††††††††††††††††††††††††† †††††††††††††††††††††† ††††††(17.40)
When ![]() †thus:
†thus:
![]() ††††††††††††††††††††††††††††††††† †††††††††††† ††††††††††††††††††††††††††††††††††††††††††(17.41)
††††††††††††††††††††††††††††††††† †††††††††††† ††††††††††††††††††††††††††††††††††††††††††(17.41)
In which
![]() ††††††††††††††††††††††††††††††††† †††††††††††††† ††††††††††††††††††††††††††††††††††††††††††††††† ††††††(17.42)
††††††††††††††††††††††††††††††††† †††††††††††††† ††††††††††††††††††††††††††††††††††††††††††††††† ††††††(17.42)
Thus:
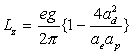 †††††††††††††††††††††††††††††††††† ††† ††††††††††††††††††††††††††††††††††††††††††††††† ††††††(17.43)
†††††††††††††††††††††††††††††††††† ††† ††††††††††††††††††††††††††††††††††††††††††††††† ††††††(17.43)
As we know eg= h
Thus:
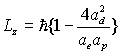 ††††††††††††††††††††††††††††
†††††††††††††††††††††††††††††††††††††††††††††††††††††††††††† ††††††(17.44)
††††††††††††††††††††††††††††
†††††††††††††††††††††††††††††††††††††††††††††††††††††††††††† ††††††(17.44)
Thus:
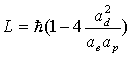 †††††††††††††††††††††††††††††††††††††††††††††† ††††††††††††††††††††††††††††††††††††††† ††††††††(17.45)
†††††††††††††††††††††††††††††††††††††††††††††† ††††††††††††††††††††††††††††††††††††††† ††††††††(17.45)
Thus:
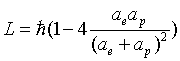 ††††††††††††††††††††††††††††††††††††† †††††††††††††††††††††††††††††††††††† ††††††††(17.46)
††††††††††††††††††††††††††††††††††††† †††††††††††††††††††††††††††††††††††† ††††††††(17.46)
Thus:
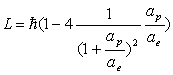 †††††††††††††††††††††††††††††††††††††††† ††††††††††††††††††††††††††††††††††† ††††††††(17.47)
†††††††††††††††††††††††††††††††††††††††† ††††††††††††††††††††††††††††††††††† ††††††††(17.47)
As we know
![]() ††††††††††††††††††† ††††††††††††††††††††††††††††††††††††††††††††††††† ††††††††††††††††††††††††††††††††††††††††††††(17.48)
††††††††††††††††††† ††††††††††††††††††††††††††††††††††††††††††††††††† ††††††††††††††††††††††††††††††††††††††††††††(17.48)
Thus:
![]() †††††††††††††††††††††††††††††††††††††††††††††††††††† ††††††††††††††††††††††††††††††††††††††††††††††††††††††† ††††††††(17.49)
†††††††††††††††††††††††††††††††††††††††††††††††††††† ††††††††††††††††††††††††††††††††††††††††††††††††††††††† ††††††††(17.49)
Based on the equations (17.3) and
(17.4), we can get the ratio of magnetic charge and electric charge inside the hydrogen
atom:
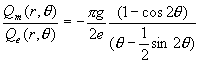 ††††††††††††††††††††††† ††††††††††††††††††††††††††††††††††††††††††††† ††††††††(17.50)
††††††††††††††††††††††† ††††††††††††††††††††††††††††††††††††††††††††† ††††††††(17.50)
For the whole north pole area which ![]() †is within
†is within ![]()
Thus:
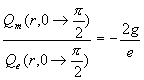 †††††††† †††††††††††††††††††††††††††††††††††††††††††††††††† ††††††††††††††††††††††††††††††(17.51)
†††††††† †††††††††††††††††††††††††††††††††††††††††††††††††† ††††††††††††††††††††††††††††††(17.51)
For the whole south pole area which ![]() †is within
†is within ![]()
Thus:
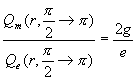 †††††††††††††† †††††††††††††††††††††††††††††††††††††††††††††† ††††††††††††††††††††††††††††††(17.52)
†††††††††††††† †††††††††††††††††††††††††††††††††††††††††††††† ††††††††††††††††††††††††††††††(17.52)
|
What
is the electron spin?
ISBN 0-9743974-9-0 Copyright © 2003 Gengyun Li All rights reserved http://www.electronspin.org |